
About the author: FRANK STEARNS is an Adjunct Professor teaching Genetics and a writing course on Science Communication. He is interested in adaptation and speciation genetics and in the history of biology. He recently finished a postdoc at Johns Hopkins University and he runs a Facebook page (Darwin’s Bulldog) that shares evolution news.
In a recent study by Ruckman and Blackmon (“The March of the Beetles: Epistatic Components Dominate Divergence in Dispersal Tendency in Tribolium castaneum”) the authors investigated the genetic contribution to dispersal in the red flour beetle Tribolium castaneum. Dispersal is an important behavior in many organisms, particularly with regard to divergence. Behavioral traits are known to have complex genetics, and dispersal in T. castaneumis no exception. The authors found there was a contribution to dispersal from a combination of additive loci and 3 epistatic loci.

Epistasis is the condition where two or more genes interact, or a gene effect is dependent on its genomic context (or genetic background). Unlike additive effects, combinations of epistatic alleles can produce effects that are different from the sum of their individual effects. For example, sign epistasis is a condition where the genotypes at two (or more) loci each increase a trait state, but when found in the same genome decrease that trait.
The term epistasis was coined by Bateson in 1907 after it was discovered in careful work done by Muriel Wheldale, one of the many women in Bateson’s early “school” of genetics at Cambridge. Using careful crossing designs and mathematical analysis she found this interesting exception to Mendel’s recently rediscovered rules of heredity.
Ruckman and Blackmon used similar classical genetics methods to elucidate the genetics of dispersal. Starting with a single wild collected population that had been acclimated to lab conditions for five years, they performed selection experiments to establish high and low dispersal lines. They used an experiment with a series of chambers, selecting for beetles that either stayed put in the first chamber or moved into another chamber over 24 hours.
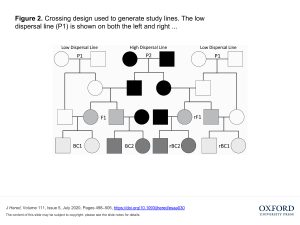
The selected lines where then used in a crossing design to estimate the genetic contributions to dispersal. Parents from the low and high dispersal lines (P1 and P2 respectively) were used to create hybrid F1 lines and reciprocal F1 lines (where male and female were switched from high dispersal to low dispersal lines) and then to establish eight backcross and reciprocal backcross lines (Figure 2 from the article).
Finally, they use a line cross-analysis (LCA) to estimate genetic architecture for dispersal. They relied on mathematical modeling to infer the most likely genetic composition of the lines, finding that the best fit came from a combination of additive loci and 3 epistatic loci.
Although epistasis is now known to be an important component of the genetic complexity underlying behavior, it has historically been left by the wayside in genetics. This is particularly true in breeding and agriculture where the emphasis has been on the rapid response to selection on additive variance. Ruckman and Blackmon suggest that it is just this rapid response that quickly depletes additive variance and leaves epistatic variance as a reservoir for future adaptation and divergence.
Epistasis was also one of the major points of disagreement between two of the giants of population genetics, Sewall Wright (who felt it was important) and Ronald Fisher (who tended to minimize and ignore its contribution). This was particularly evident in Wright’s Shifting Balance Theory. Adaptation can be viewed as the movement across a topology, like a topographic map. While Fisher essentially ignored epistasis and envisioned a “smooth” landscape with a single peak, Wright included epistasis in his model and generated a “rough” landscape with peaks of high fitness and valleys of low fitness. Here Wright suggested that the “problem” of adaptation involved the crossing of valleys of low fitness in order to climb peaks of high fitness, moving to higher and higher peaks. He suggested that small semi-isolated populations would be able to go through stages of drift and selection in order to traverse these valleys. Ruckman and Blackmon implicitly engage with this work and seem to suggest epistasis may serve as a reservoir for variation to aid this process.
The authors conclude that epistatic variance may have originally contributed more to dispersal than they found, being converted to additive variance during the course of their selection experiments. They suggest future studies repeating this design but varying population size. The expectation would be that smaller populations (like those proposed by Wright) would more rapidly convert epistatic variance to additive variance. This can give us insights into the role epistasis plays in the process of divergence and the transition to new adaptive peaks.
References



