
About the Blog Author: Marcelo Barreto Filho has a bachelor’s degree in Biological Sciences at the Federal University of São Carlos (UFSCar). He has a MS in Ecology and Natural Resources from UFSCar-PPGERN, with a study abroad period in the Evolutionary Studies Institute at the University of Witwatersrand, Johannesburg, South Africa. During his MSc, Marcelo developed research in Programmed Cell Death (PCD) on coccoid green microalgae and its evolutionary ecology fitness consequences. Currently, Marcelo is a PhD student in The University of Alabama at Birmingham in the Morris Lab working on the co-evolution of heterotrophic bacteria and phytoplankton in face of environmental change. His project’s main objective is to quantify the evolutionary and ecological stability of black queen interactions (BQ) impacting the biogeochemical cycles of nutrients in aquatic ecosystems.
Why do individuals spend time and energy taking care of other’s offspring?
In beehives, why are workers specialized to defend conspecifics at expense of their own reproductive success?
What can these altruistic behaviors teach us about the evolution of cell suicide in unicellular lineages?
Let’s start with the first two questions. Altruistic behaviors were a headache to Darwin (1859). He considered social insects a potentially “insuperable difficulty” for his theory of natural selection. Fitness is the number of descendants in the next generation – so how can evolution by natural selection explain altruism?
To understand, we need to think about genetics. The degree of kinship between parents and their direct descendants is 50%. The same degree of relatedness can be observed, on average, in complete siblings. In a population, the average degree of kinship is known as Wright’s coefficient of relatedness (Wright, 1917), or the ‘r’ value, and can be calculated as the percentage of shared genes in any two paired individuals in the population.
Haldane (1955) and Fisher (1930) suggested that natural selection favors the spread of genes making for altruistic behaviors in small populations. In such populations, relatedness is higher and ‘non-random associations between genotypes’ would appear – an essential condition for an altruistic behavior to evolve by selection. It was not until 1964, however, that Willian Hamilton (1964) developed a genetic equation using Wright’s coefficient as well as Haldane and Fisher’s thoughts, to demonstrate how an allele favoring altruistic behavior could be spread through the population. Hamilton’s rule (1964) is expressed by the following equation:
rB – C > 0
where r is the coefficient of relatedness between the actor and the recipient of the altruistic behavior. B is the benefit brought to the recipient by the altruistic act and C is the cost imposed on the actor by the altruistic behavior, where B and C are expressed in units of surviving offspring. Altruism may evolve by natural selection when rB > C.
Hamilton (1964a) introduced the concepts of direct, indirect, and inclusive fitness. Direct fitness is the gain from personal reproduction, while indirect fitness is the extra gain thanks to helping out relatives to survive. Inclusive fitness is the sum of direct and indirect fitness and can be interpreted as the total genetic contribution of an individual to the next generation. Altruism, then, may evolve if individuals are related and the indirect gain is larger than the costs associated to the altruistic behavior. The term ‘kin selection’ was coined by John Maynard Smith (1964) by using Hamilton’s rule in an attempt to explain the evolution of costly behaviors.
What does altruism (and kin selection) have to do with cell suicide in unicells? In his 1964’s paper, Hamilton (1964b) described the classic evolutionary series of the green microalgae Volvox (Fig. 1) as an example of kin selection. Volvox (and related Volvocaceae algae) are colonial microalgae ‘composed’ by unicells which are similar to its unicellular relative, Chlamydomonas.
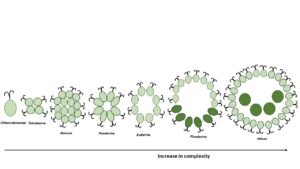
We have to think as a unicellular microalga living in an unfriendly environment full of predators: small individuals are eaten easily by predators (i.e., zooplankton). Predation was playing an important evolutionary pressure for group formation. The existence of different species in the same environment, however, was a problem to group formation as different genotypes would preferentially invest on themselves fitness rather than the group (Durand et al., 2016). To overcome this, a mechanism which promotes group homogenization was required.
How was group homogenization achieved? According to a rumor, the British Biologist JBS Haldane, would have said once in a pub: “I would lay down my life for two brothers or eight cousins”. If you remember about Hamilton’s equation discussed earlier, this is because the higher relatedness between brothers than cousins. Unicellular microalgae, of course, do not ‘think’ like Haldane. However, would unicells somehow die to ‘save’ their relatives? Recent research on Chlamydomonas reinhardtii has suggested this may be true. When exposed to heat shock, this microalga undergoes programmed cell death – PCD or more colorfully cell suicide – releasing products into the environment that not only help relatives to survive (Durand et al., 2011), but also inhibit the growth of unrelated species (Durand et al., 2014). My current research on another green microalga, Ankistrodesmus densus, showed similar findings after induction of PCD and application of PCD-supernatants to A. densus and related species (not published). These species-specific products increase the genetic relatedness and favor multicellular aggregate formation.
Inclusive fitness and kin selection provided an elegant solution by how selection can promote the evolution of altruism in multicellular as well as unicellular lineages and would have been greatly helpful as good analgesic to Charles Darwin’s biggest headache.
References
Fisher, RA (1930) The genetical theory of natural selection. Clarendon Press.
Haldane, JBS (1955) Population genetics. New Biology 18:34-51.
Smith, J (1964) Group Selection and Kin Selection. Nature 201: 1145–1147.
: https://www.jstor.org/stable/2456273?seq=1



